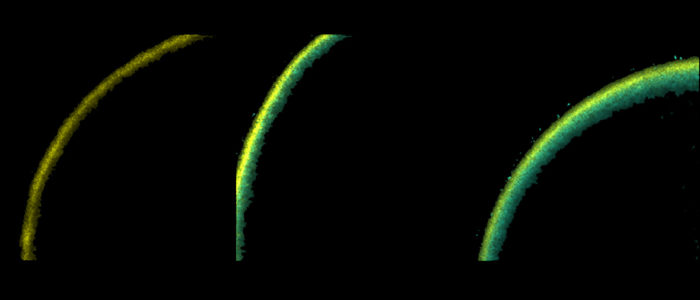Trudeau Lab has a new opening available for a postdoctoral fellow. For more information, click here.
The focus of the work in my lab is on voltage-activated potassium channels in the hERG-family of ion channels. hERG channels are known for their role in cardiac electrophysiology where they help to repolarize cardiac action potentials. Genetic mutations in hERG channels are linked to the long QT syndrome, a cardiac rhythm disorder that is characterized by a prolonged QT interval on an electrocardiogram and is a risk factor for sudden cardiac death. hERG channels are also of significance because the unintentional side-effect of many therapeutic drugs is a prolonged QT interval and risk of sudden death, which is primarily due to the inhibition of hERG channels. For these reasons, hERG channels are extremely important for basic cardiac physiology and in pathophysiology of disease.
Recent work in my lab has focused on the molecular mechanism that underlies the opening and closing (gating) behavior of hERG channels. hERG channels have very unusual kinetics that tune them for their role in the heart. In particular, the channels have slow kinetics of closing (deactivation). We have investigated the mechanism of slow deactivation in these channels recently. One project in the lab that we are focused on found that the N-terminal region of the channel regulates the deactivation of the channel. The N-terminal region contains an “eag domain” which comprises a PAS domain and a PAS-Cap domain and is necessary for regulation of deactivation. In an interesting development, the N-terminal PAS domain regulates channel gating by interacting directly with the C-terminal Cyclic Nucleotide Binding Homology Domain (CNBHD) of the channel. This interaction and other molecular interactions in hERG channels are the subject of projects in the lab.
To carry out our studies, we use several different technologies.
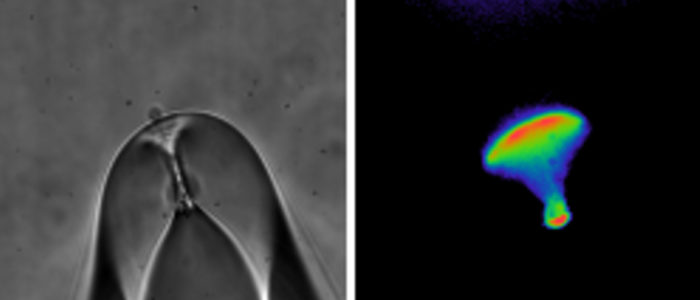
One of the powerful technologies we use is dual electrophysiology to measure channel function and fluorescence spectroscopy to measure channel structural movements. Students and postdocs in the lab will learn two-electrode voltage-clamp, whole-cell patch-clamp and excised, inside-out patch-clamp electrophysiology methods. Each of these methods can be combined with fluorescence spectroscopy to measure simultaneous changes in ion channel structure and function. For instance, Patch-Clamp Fluorometry (PCF) combines excised patch recording of ionic currents with fluorescence spectroscopy measurements from fluorophore-labelled ion channels in a patch of membrane.

For fluorescence measurements we have used CFP and YFP (and derivatives) as donor and acceptors in FRET experiments. Recently the lab is interested in using amber codon suppression technology to introduce different non-canonical amino acids (ncAAs) into ion channels. The ncAAs serve as chemical handles that can undergo modifications that report new structural information about ion channels. For instance, we have been studying the fluorescent ncAA L-ANAP incorporated into hERG channels as a reporter of structural changes in hERG in response to changes in membrane voltage.
In addition, the lab is using stem cell derived cardiomyocytes to study native hERG channels and the cellular effects of modifications to the hERG current with voltage-clamp and current-clamp methods. We investigate how familial LQTS-associated mutations in hERG alter the hERG current and use biophysical knowledge of hERG to ameliorate or repair defects in LQTS-hERG channels. We are also interested in the mechanism of drug inhibition of hERG, as blockade of hERG channels in the heart is associated with acquired LQTS.